What are the most common pitfalls in human semen analysis?
There are many basic problems/pitfalls in basic semen analyses. Even in many ESHRE accredited laboratories that I visited during the last five years one or more of these pitfalls were evident:
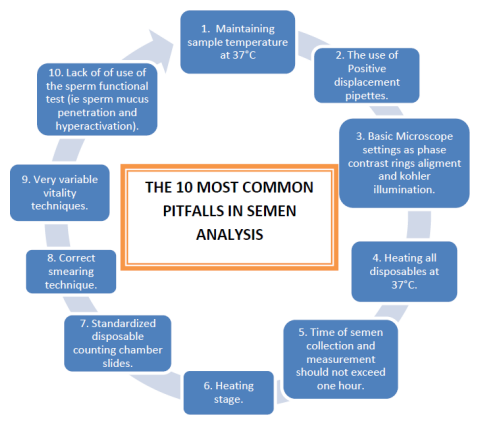
1. Maintaining the temperature of sample at 37°C at all times. Soon after collection as well as during measurement of sperm motility (see later).
2. Out of some 40 andrology laboratories in 25 countries visited in five years, only three were using a positive displacement pipette for aspirating sperm for sperm concentration determination. Most staff in these laboratories never heard of a positive displacement pipette and actually uses the normal laboratory pipettes which are negative displacement pipettes. With viscous media such as semen these are prone to collect tiny air bubbles not visible to the eye but moreover sperm stick to the surface and result in inaccurate sperm concentration determination.
3. Basic microscope settings for both bright field and positive phase contrast optics are simply not adhered to. The most important among these are critical and Köhler illumination. It is simply not realized by analysts that it is not just the focus on the object that is crucial but also focusing of the beam of light on the object (sperm) and at the same time maintaining an even background illumination (Köhler). This is essential for CASA.
4. Heating of microscope slides, pipette tips and any other consumable that will make contact with the semen sample to 37°C. It does not help for example to heat the microscope slide to 37°C but use a pipette tip at room temperature. Human sperm is sensitive to temperature changes and temperature shock can occur even with small gradients.
5. Actual time of semen collection and measurement of key sperm functional aspects shortly after liquefaction but before one hour after ejaculation. Both sperm motility and sperm vitality should be measured within one hour of collection. These two parameters decline quite rapidly after one hour particularly in poor quality samples.
6. A temperature controlled microscope stage is missing in most laboratories for sperm motility measurement. Particularly for CASA this is essential! Why? The cut-off points for progressively sperm is determined by actual speed of swimming (VCL). The metabolic rate of sperm at 37°C and 20°C (typical room temperature of many laboratories) are hugely different and sperm will sperm considerably slower at even 27°C compared to 37°C.
7. Not using standardized slides for concentration/motility measurement. In this context most laboratories use the so called cover slip/slide method for manual assessment of sperm motility. However, the motility is highly variable in different parts of the slide and counting motile/immotile just in the central areas will usually provide an over-estimation of sperm motility percentage determination. Fixed chamber slides such as Leja provide more consistent data from one field to the next, but motility can be over or under estimated if not carefully controlled (Segre Silberberg effect). The Makler chamber does not appear to be accurate when sperm concentration is low.
8. The standard method of making correct sperm smears are not adhered to in many laboratories. Instead of dragging the semen behind the slide the semen drop is pushed and the sharp edge of the slide can break up sperm.
9. Technique for vitality with eosin-nigrosin vary variable in terms of different techniques employed. Also here very poor adherence to maintaining solutions, pipette tips and slides at 37°C.
10. Just using basic semen parameters to try and define the fertility potential of a semen sample instead of starting to also incorporate sperm functional tests such as sperm cervical mucus penetration and hyperactivation.
Gerhard van der Horst (PhD, PhD)
Senior Consultant
MICROPTIC S.L.




Leave A Comment