Epigenetics and lifestyle: new perspectives for future diagnostics
Have you ever wondered why some people are more prone than others to suffer from obesity, diabetes or cancer? The most probable answer is thinking that these diseases are related to genes that parents transmitted to their progeny, which at the same time, were also transmitted by their grandparents. But what you probably have not been told is about the importance of the lifestyle of parents, since it has been recently connected to the inherited health of their progeny.
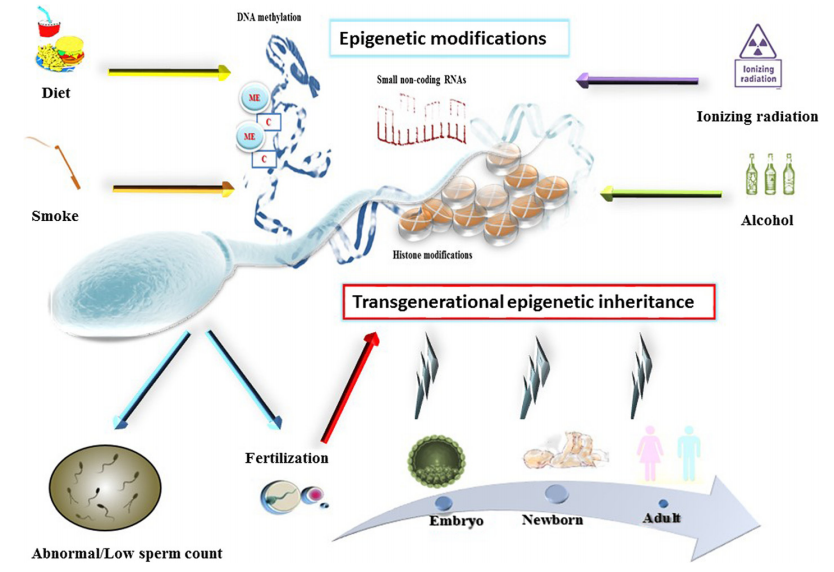
In humans, stress, changes on nutrition, as well as a non-healthy lifestyle (smoking, alcohol consumption and sedentarism) is resulting in a variety of neuroendocrine, immune and behavioural responses (Refs. 1-4). But also, these factors are affecting on reproductive potential of grown-up people. The crucial role that modifiable lifestyle factors play in the development of adult infertility has generated a growing interest in this field, especially because recent epidemiological studies have evidenced that the lifestyle of one generation can modify the risk of developing chronic diseases or idiopathic infertility in subsequent generations through so-called parental effects (Refs. 5, 6).
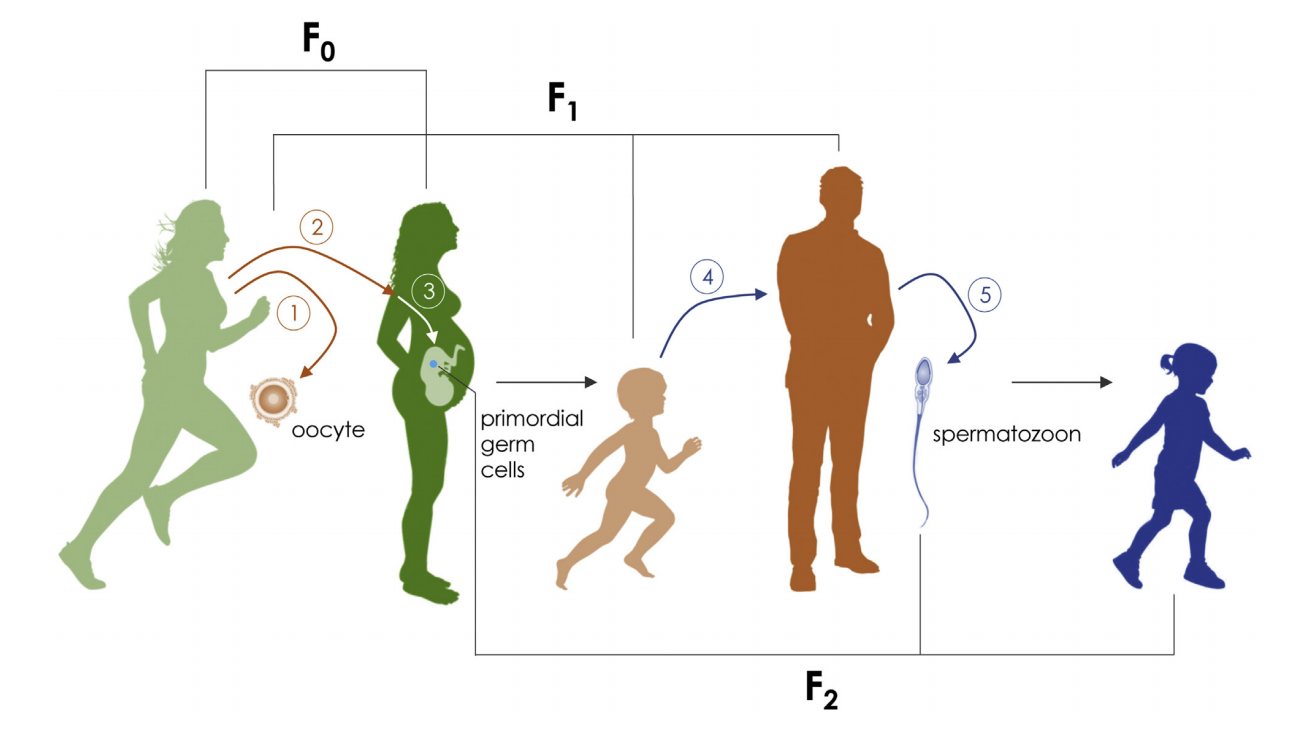
Studies of human populations and animal models suggest that a father’s experiences such as diet or environmental stress can influence the health and development of his descendants (Refs. 7, 8). As an organism grows and responds to its environment, genes in its cells are constantly turning on and off, with different patterns of gene expression in different cells.
On the same way that happens with somatic cells, germ cells are also subjected to the effect of environmental factors. This phenomenon, called “Environmentally-driven epigenetic modifications of gametes”, provide a potential molecular basis to explain the transmission of developmental plasticity across generations, as well as a mechanism to understand “missing” heritability factors observed with certain diseases.
A very recent publication on Nature Communications (Ref. 9) has demonstrated that, in C. elegans (a nematode worm), epigenetic information carried by parental sperm chromosomes can cause changes in gene expression and development in the offspring. Despite epigenetic changes do not alter the DNA sequences of sperm genes, these could involve chemical modifications (i.e., changes in methylation patterns) to either the DNA itself or to the histone/protamine proteins with which DNA is packaged. These modifications or “marks” could change gene expression, turning genes on or off.
In humans, nutritional status and physical activity levels were also associated with dynamic epigenetic changes in spermatozoa (Refs. 10, 11), providing evidence that lifestyle factors prior to conception can modulate the health of the offspring through gamete epigenetic inheritance in humans as well. At a more specific level, it has been suggested that DNA methylation defect could influence on the sperm fertilizing capacity and the embryo development (Refs. 12,13). Likewise, imprinting errors of primordial germ cell lines has been associated to abnormal spermatogenic maturation processes in adult males (Refs. 14, 15).
All these evidences indicate that gametogenesis is a very sensitive process that could be easily modulated by multiple epigenetic factors such as nutrition, exercise, endocrine disruptors, as well as traumatic stress (Ref. 5). Thus, further evaluations should be performed about whether lifestyle could influence on the welfare of future generations, since our children are the ones who will pay for the consequences of the decisions of our today.
Núria Medarde, PhD
Biologist
MICROPTIC S.L.
References
- https://www.ncbi.nlm.nih.gov/pubmed/27208688
- http://www.ccsenet.org/journal/index.php/jas/article/view/71761
- https://www.ncbi.nlm.nih.gov/pubmed/28929508
- https://www.ncbi.nlm.nih.gov/pubmed/25552409
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6034033/
- https://www.ncbi.nlm.nih.gov/pubmed/28410129
- https://www.ncbi.nlm.nih.gov/pubmed/30474562
- https://www.smithsonianmag.com/science-nature/dads-pass-more-genetics-their-sperm-180969760/
- https://www.nature.com/articles/s41467-019-09141-w
- https://www.ncbi.nlm.nih.gov/pubmed/25864559
- https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-018-0446-7
- https://www.eshre.eu › Annual-meeting › Course-10 (DNA methylation in sperm: patterns, regulation and inheritance. Mehdi Benchaib, MD, PhD)
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3583177/
- https://www.ncbi.nlm.nih.gov/pubmed/16608903
- https://www.eshre.eu › Annual-meeting › Course-10 (Imprinting in sperm of men with abnormal semen parameters. Cristina Joana Marques, PhD, Alberto Barros, MD, PhD, Mário Sousa, MD, PhD)




Leave A Comment