Tricks for optimizing the analysis of sperms with the SCA

1. The presence of bubbles inside the counting chamber may alter the results obtained by your SCA.
This occurs when:
a) Non-optimal counting chambers are used for automatic analysis.
b) The counting chamber is not filled in correctly.
– Micropipettes must be correctly calibrated to prevent air bubbles during sample aspiration/release.
– Counting chambers must be filled in progressively and in one step. It’s recommended to press slowly the plunger button of the pipette allowing a continuous and homogeneous sample release.
2. Use the Intelligent Filter.
The intelligent filter option in the “Configuration” menu allows the distinction of sperms (cells with tail) from debris/other cells that have no tail. The activation of that setting offers a higher accuracy throughout the calculation of sperm concentration, for the detection of artefacts that could be erroneously classified as sperms is omitted.
NOTE: When working with a USB Basler acA-1300 camera, it’s recommended to change the resolution of the “Capture” setting to optimize the sensitiveness of the analysis:
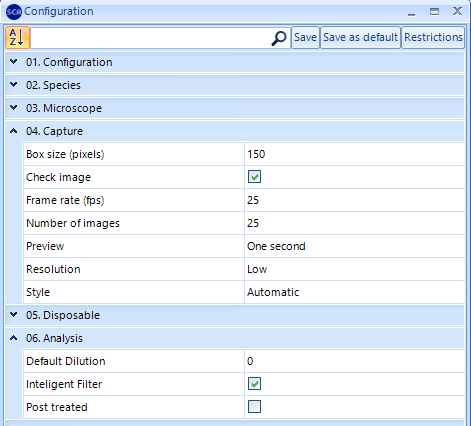
SCA 6.3 version: Select “Low” Capture Resolution
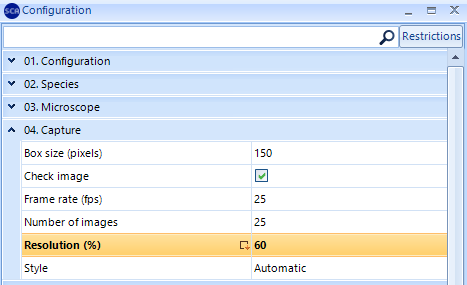
SCA 6.2 version: Change the Resolution from 100% to 60%
3. Check the “Comparison” option for getting robust results.
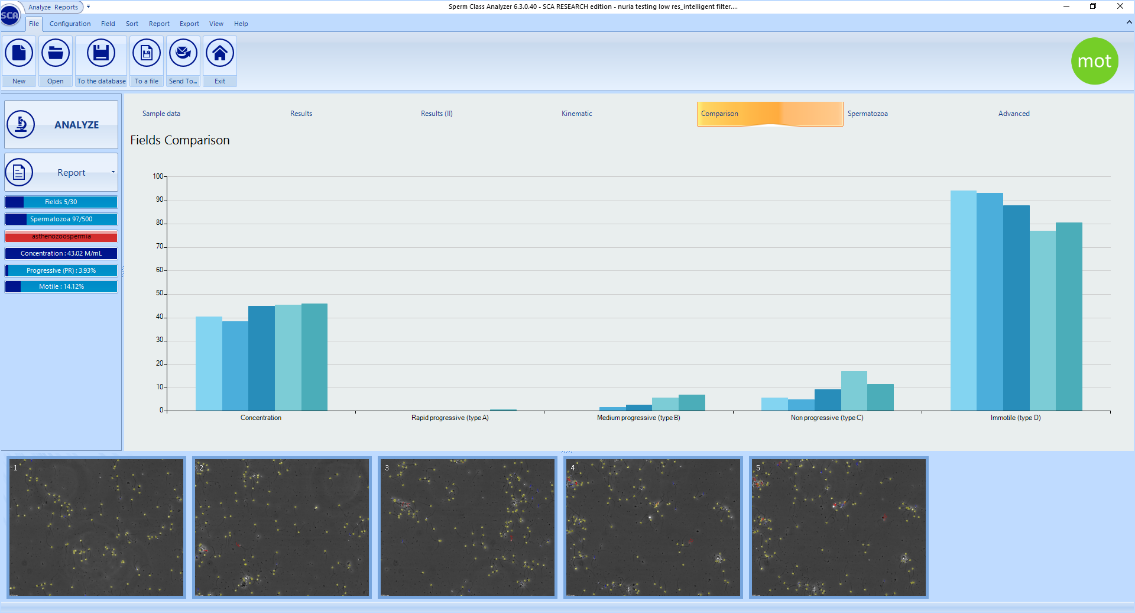
The “Comparison” graph allows to check the homogeneity of the captured fields. When fields are homogeneous, these are shown in blue colour and concentration values are similar in the graph:
However, when fields show very different concentrations (i.e. due to the presence of aggregations or agglutinations), these are shown in red and the graph for concentration is heterogeneous:
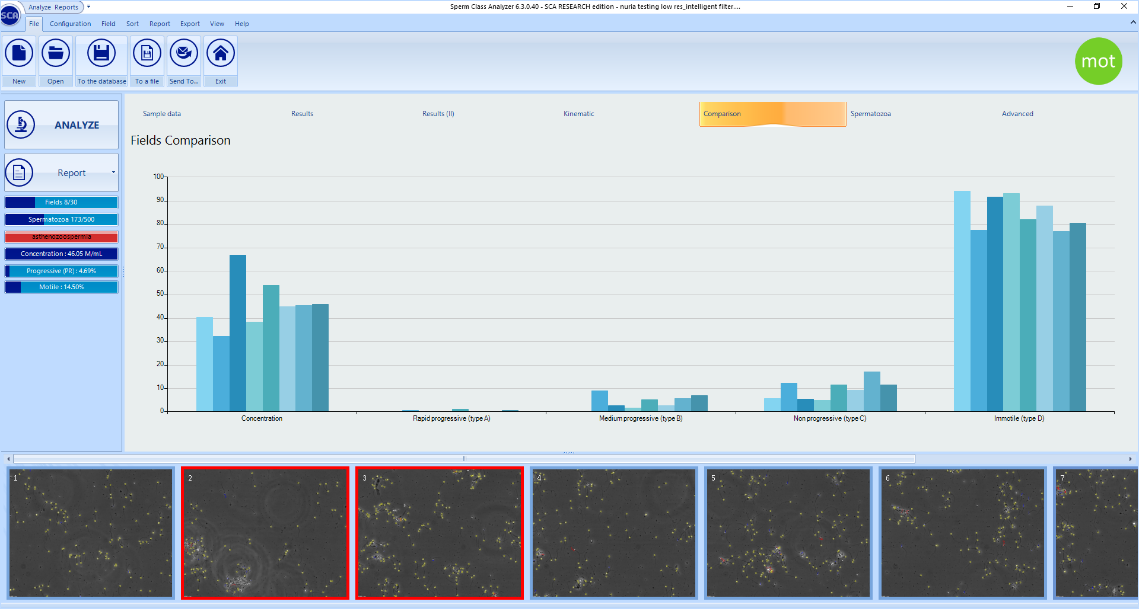
In those latter cases, it’s recommended to delete the dissimilar fields shown in red and capture more fields (if required) to get more reliable results.
1. Getting an optimal staining.
Staining protocols have been standardized considering specific laboratory environmental conditions (50% relative humidity and temperature to 21-23°C). But, since many laboratories are not working under these conditions, staining procedures must be adapted to every specific laboratory conditions before performing an automatic analysis.
Intrinsic seminal factors may also hinder the automatic detection. Viscosity and seminal proteins commonly result in a dim and noisy background once semen smears are stained. In these cases, a simple sperm washing is recommended to get a clearer background and a higher contrast between the distinct parts of sperm cells.
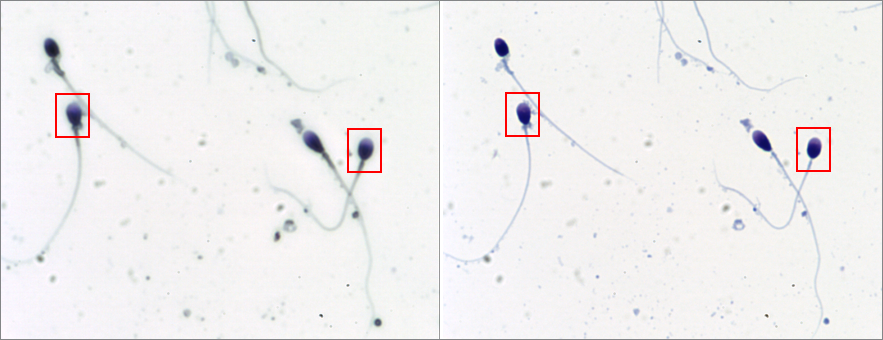
2. Capturing accurately at high magnifications.
Morphological analysis at 100x but also at 60x require the use of a mounting medium (i.e., Eukitt, DPX, or similar) and a coverslip to get optimal focus and a good contrast of the different sperm head regions and vacuoles. If smears are not mounted, overestimation of the size and non-realistic results could occur.
Non-mounted (left) vs Mounted (right) slide at 60x:
Nuria Medarde, PhD
Biologist
MICROPTIC S.L.



Leave A Comment