Are microscope settings and stain selection really important in sperm quality assessment?
How good are you at using your routine or research microscope in the lab or in the Andrology setting? If you cannot immediately answer the following three questions, then you are not using the microscope correctly and potentially miss out on many fine microscopic size details. So here they come:
- Do you know how to setup your microscope for critical and Köhler illumination every day before you start?
- Do you know what ALL the inscriptions on a typical objective lens represent apart from the magnification? For, example, what does the 0.65 inscription represent on a 40x objective and is it important?
- What does the term resolution refer to?
Do not feel bad if you could not answer any of these or only some BUT if you routinely use a microscope rectify these shortcomings.
All the above principles have been reviewed in three blogs on the Microptic SL webpage dealing with Microscopy (Part I, part II and part III). Accordingly, please refer to these Microscopy Blogs. However, I will briefly explain the important principles associated with the above bullets.
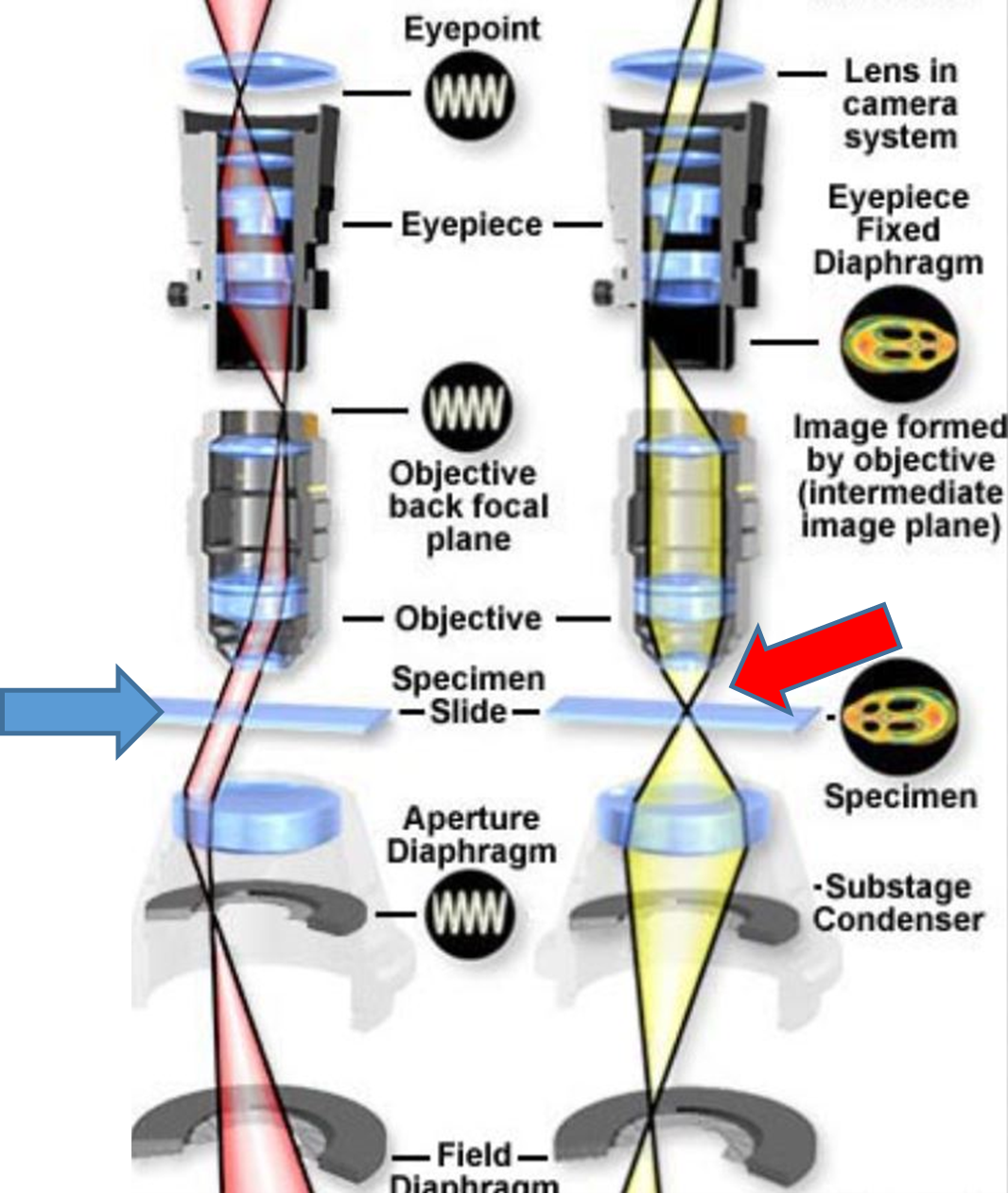
Fig. 1 A: Uniform illumination / Critical illumination: Blue arrow (left) shows parallel light waves (providing uniform illumination) and red arrow (right) shows light waves sharply focused on the specimen (object).
Firstly, critical and Köhler illumination: The average microscope user believe that it is sufficient to turn up the light to a level just to clearly view and focus the object (sperm) and that’s it. No, it is not only the object (swimming sperm, stained smear) that needs to be focused but just as important the light beam from the lamp/LED source and via the condenser lens that must be sharply focused on the object (specimen). So if correctly set the pinpoint beam now provides n number of photons that interact with the image and ALL of it will contribute to the image. If there is just slight under or over focusing of the light beam on the image, then many photons are lost as they have not interacted with the image. So in total less information can be collated/collected by the objective lens and the image sharpness will not be perfect. You may not realize this just viewing with the naked eye but it can make a huge difference when using image analysis such as SCA motility, following motile sperm or detecting immotile sperm or differentiating the different parts of a sperm during morphology analysis.
So, what about Köhler illumination? In 1893, Prof August Köhler introduced the revolutionary concept of uniform/even illumination at Jena, Zeiss, Germany (Figs.1A and B). When the light beam is perfectly focused on the image (critical illumination) then those parts of the beam not interacting with the image pass as parallel waves and that produces even or uniform background illumination. Imagine that your background illumination has different gradients of grey instead of all parts of the background having uniform illumination, then the software may pick up that unevenness as a particle(s) (false or ghost image) for example. So the two aspects critical and Köhler illumination go hand in hand and the microscope can be set for this within half a minute (refer to the previous Blogs and also to the SCA manual where this is explained).

Fig. 1 B: Photograph of Prof Köhler
What about the 0.65 inscription on the 40x objective and resolution? That refers to the numerical aperture of that lens(NA) and actually tells you that when you have set the illumination uniformly the 40x lens will yield a NA of 0.65 and that relates to the resolution of the lens. This is very important and informs us of the minimum distance that we will be able to distinguish one object from the other Accordingly, for a 40x lens the resolution under ideal illumination will be about half a micrometre.
Once the microscope setup is perfect the specimens can be viewed under optimal conditions and analysed by image analysis such as SCA. Let us discuss viewing of slides stained for morphology and the first question that arises is does it matter which stain is used and what the purpose of staining is? There are many stains and stain combinations for human and animal sperm. Chenoweth and Norton listed some 32 stains for a wide range of domestic animal applications. Let us first discuss some applications and then decide what should be used. For human sperm PAP and closely related Shorr and Diff-Quik have been extensively used. Recently many IVF centres and andrology labs worldwide also started using SpermBlue (SB). The three main reasons for using SB are the simplicity of the technique (once the smear is dry it takes 1 minute to complete staining), it is isosmotic and isotonic to sperm (does not cause shrinking or swelling) and stains the acrosome, head, midpiece and tail in different hues of blue. In contrast more than 90% of other stains cause either swelling of sperm (Diff-Quik) or shrinking (PAP) and many stain background particles. The other advantage of SpermBlue is that it seems to work for all animals (insects, other invertebrates and apparently all vertebrates) and are useful when comparisons are made for different breeds and species.
The nigrosine-eosin stain (NE) combination (Percentage vitality) has also been extensively used for human and animal sperm to distinguish between “live” and “dead” sperm but also used for morphology. Recently, Microptic SL provided a NE-based stain, BrightVit, that has been prepared in a hypo-osmotic medium and can also be used for the HOS-test. Other stains commonly used in the Animal industry is Gentian violet, SperMac (differentiates acrosome, head and midpiece in three colours) and Silver staining to differentiate the different sperm components more clearly (domestic animal market). Other triple staining techniques have been used consisting of Trypan blue, Bismarck Brown and Rose Bengal which differentiates live-dead and acrosome reacted sperm. Toluidine Blue and Aniline Blue have been used for sperm fragmentation and chromatin maturity.
Finally, which stain is ideal for Computer Aided Sperm Morphology analysis (measure sperm morphometrics, the percentage normal sperm morphology, where cut-off points are known, as well as the sperm indices determine TZI and MAI)?
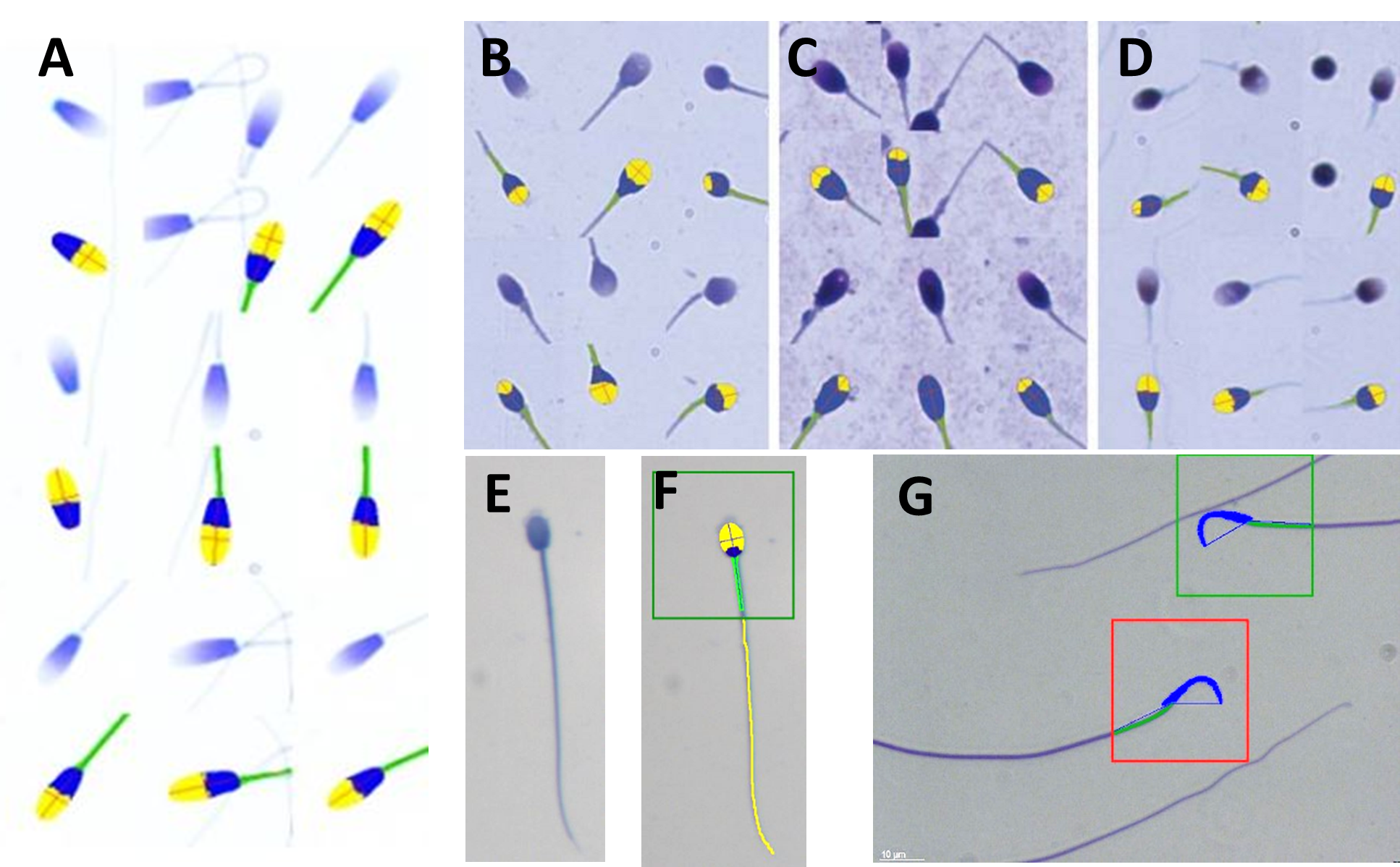
Fig. 2. A to G represent sperm of different species stained with SpermBlue (SB) (except C and D) but also the automated SCA CASA morphology analysis recognizing the acrosome, rest of head and midpiece and rest of tail in F. A = Horse sperm and each stained sperm shown at top and immediately below the SCA analysis of that sperm; Human sperm stained with SpermBlue (B); with Diff-Quik (C – swollen and acrosome poorly represented); with PAP (D – shrink). In all three B, C and D sperm stained on top and immediately below analysis with SCA morphology module; E = Hyrax sperm; F = Sperm in E analysed by SCA including the tail and in green block indicating it is normal; G = Wistar rat sperm at top normal in green block and bottom, macrocephalic in red block (abnormal).
In summary: Yes, microscope settings such as critical illumination are very important and must be perfectly set to be able to produce an image that is sharp (high resolution) with an even background illumination. However, if the staining procedure interferes with the normal live dimensions of the sperm and if the stain provides background noise (debris) it largely defeats the purpose. Figures A, B and E-F provide ideal consisting staining and CASA analysis of SB stained sperm.
Prof Gerhard van der Horst (PhD, PhD)
Senior Consultant
MICROPTIC S.L.




Leave A Comment